Whole Exome Sequencing (WES)
Whole Exome Sequencing (WES) is a state-of-the-art genetic technique that specifically targets the coding regions of the genome, known as the ‘exome’. These regions account for only around 1-2% of the entire human genome but are responsible for the majority of disease-causing genetic mutations. WES allows scientists to identify rare or novel genetic variants, facilitating more accurate diagnoses of inherited diseases, enhancing cancer treatment, and supporting the development of personalised medicine. This method is widely employed in both medical research and clinical practice to uncover the genetic underpinnings of diseases, playing a crucial role in advancing healthcare.
Click to read this article :What is genetic testing and why should it be done
Definition of Whole Exome Sequencing ( WES )
Whole Exome Sequencing, or WES, is an advanced genetic technique utilised to sequence all the protein-coding regions of the genome, referred to as the ‘exome’. The exome comprises genes that produce functional proteins and accounts for only approximately 1-2% of the entire human genome. However, the majority of genetic mutations that cause diseases occur within these coding regions. In the WES process, DNA extracted from a patient sample is initially fragmented, followed by the selection and sequencing of the coding regions, or exome, to identify mutations and genetic variations.
Importance of Whole Exome Sequencing in Medicine and Genetics
WES plays a pivotal role in medicine and genetics, enabling researchers and clinicians to accurately identify genetic alterations that may lead to inherited diseases and cancer. Some key benefits and applications of WES include:
- Accurate Diagnosis of Genetic Diseases: WES assists in diagnosing rare inherited conditions that may be undetectable using conventional diagnostic methods. This technique facilitates the identification of rare genetic mutations, enabling more effective treatments.
- Personalised Medicine: As genetic mutations vary among individuals, WES allows clinicians to provide more tailored treatments. This method helps determine therapies that best respond to an individual’s genome, particularly in cases of cancer and complex diseases.
- Research and Development of New Treatments: WES is extensively employed in genetic research, allowing scientists to investigate the roles of various genes in disease development and to develop novel treatments based on this information.
- Reduced Cost and Time Compared to WGS: While Whole Genome Sequencing offers more comprehensive data, WES, by concentrating on crucial coding regions, provides a more cost-effective and rapid approach for many research and clinical applications.
This technique is widely utilised in clinics and research laboratories to identify the genetic causes of diseases and enhance healthcare. It continues to play a vital role in the future advancements of genetic science and medicine.
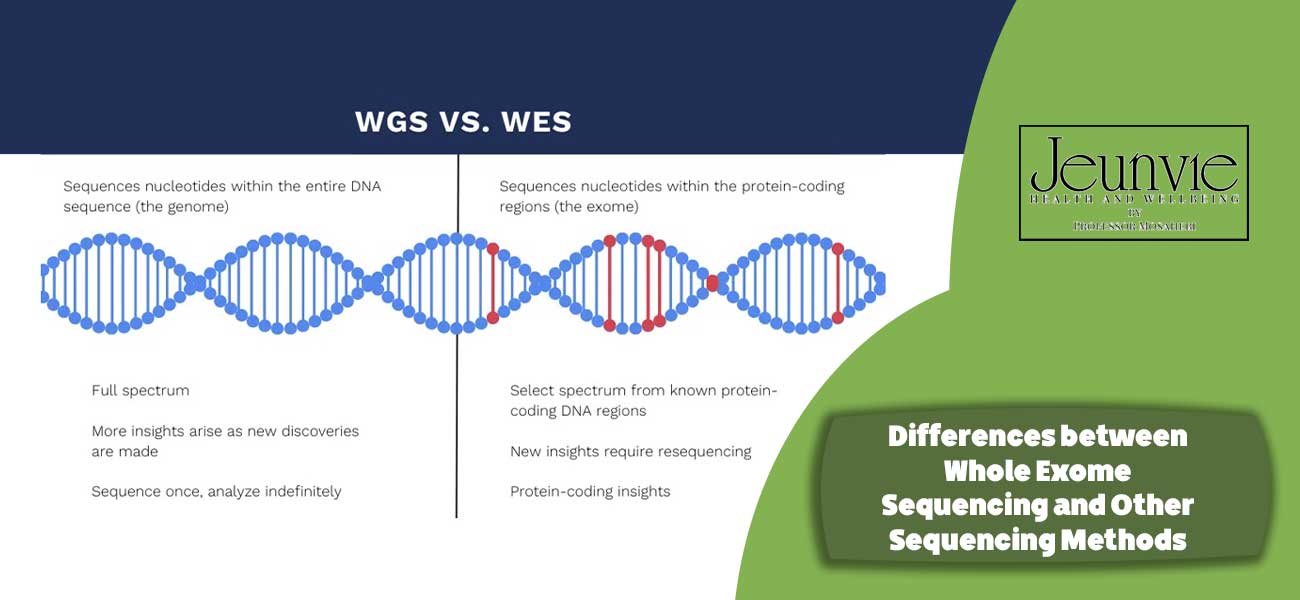
Differences between Whole Exome Sequencing and Other Sequencing Methods
Whole Exome Sequencing (WES) and Whole Genome Sequencing (WGS) are two primary methods for sequencing the genome, each with its own unique characteristics and applications. Here, we will explore the key differences between these two methods, as well as the advantages and disadvantages of WES compared to other approaches.
Differences between WES and Whole Genome Sequencing (WGS)
- Sequencing Scope:
- WES: This method sequences only the coding regions of the genome, known as the exome. These regions constitute approximately 1-2% of the entire genome and include genes that produce functional proteins.
- WGS: In contrast, WGS sequences the entire genome, encompassing both coding and non-coding regions. This method provides more comprehensive genomic information, including regulatory regions, repeats, and other genetic elements.
- Data Volume:
- WES: Generates a smaller data volume compared to WGS, thereby simplifying the analysis process.
- WGS: Produces a significantly larger data volume due to the sequencing of the entire genome, necessitating more storage and processing capabilities.
Advantages and Disadvantages of WES Compared to Other Methods
Advantages:
- Focus on Coding Regions: Given that most disease-associated mutations occur in coding regions, WES can be highly effective in identifying these mutations.
- Reduced Cost: WES is generally more cost-effective than WGS, particularly when focusing on specific inherited diseases and genetic variations.
- Faster Analysis: Due to the smaller data volume, the time required for analysis and interpretation of results is typically shorter.
Disadvantages:
- Exclusion of Non-Coding Regions: WES does not include non-coding regions, which may play crucial roles in gene regulation and disease development. This can result in the omission of important information.
- Limited Coverage: WES may not completely cover all coding regions, especially in cases of low-quality DNA samples or when analysing regions with complex sequences.
- Challenging Result Interpretation: Identified mutations may not be easily interpretable in some instances, particularly in unfamiliar coding regions, and may require further analysis.
In general, the choice between WES and other sequencing methods depends on the type of research, clinical objectives, and financial and time constraints. WES, with its focus on important coding regions, is a compelling option, especially for diagnosing inherited diseases. However, in specific circumstances, WGS or other methods may prove more advantageous.

Applications of Whole Exome Sequencing (WES)
Whole Exome Sequencing (WES)
is an advanced technique with extensive applications in various medical and genetic fields. Below, we explore some of its primary applications:
Diagnosis of Genetic Diseases
WES is particularly effective in identifying mutations that are responsible for hereditary and rare diseases. This method assists clinicians and researchers in:
- Identifying Specific Mutations: By analysing the exome, rare and unusual mutations that may be linked to specific diseases can be identified. This is especially important for conditions with ambiguous clinical presentations.
- Diagnosing Complex Diseases: Many diseases, such as rare syndromes, can arise from alterations in multiple genes. WES can help identify changes across these genes, enabling clinicians to provide a more accurate diagnosis of the disease.
- Prevention and Disease Management: By identifying hereditary mutations early, clinicians can design better prevention and management strategies for patients. This empowers patients and their families to make more informed decisions regarding healthcare.
Cancer Diagnosis
WES plays a vital role in the diagnosis and management of cancer. This method aids clinicians in:
- Identifying Cancer-Specific Mutations: WES can help detect genetic mutations associated with various types of cancers. This information assists clinicians in determining the type and stage of cancer and selecting appropriate treatment options.
- Personalised Treatments: By leveraging data from WES, clinicians can develop specific and targeted treatments for patients. These therapies are often tailored to the patient’s genetic profile and the identified mutations, making them potentially more effective than standard treatments.
Personalised Medicine
WES is recognised as a key tool in personalised medicine. This technique enables clinicians to:
- Design Genome-Based Treatments: By accurately understanding a patient’s genetic profile, WES can assist clinicians in designing more effective treatments. These may include drugs that specifically target the identified mutations.
- Predict Treatment Response: Using information derived from WES, clinicians can anticipate which treatments are likely to be most effective for each patient, thereby helping to minimise adverse effects.
Genetic Research and Evolution
WES also has significant applications in research and genetic studies:
- Population Genetics Studies: WES can help researchers investigate genetic diversity within different populations. This information contributes to a better understanding of ethnicities, common genetic disorders, and evolutionary trends.
- Research on Species Evolution: By utilising WES, researchers can analyse the genetics of different species and identify genetic changes that have led to their evolution. This can enhance our understanding of the evolutionary history of species and biological mechanisms.
In conclusion, WES is a versatile tool in medicine and genetic research, playing a significant role in the diagnosis, treatment, and investigation of genetic diseases and complex conditions.
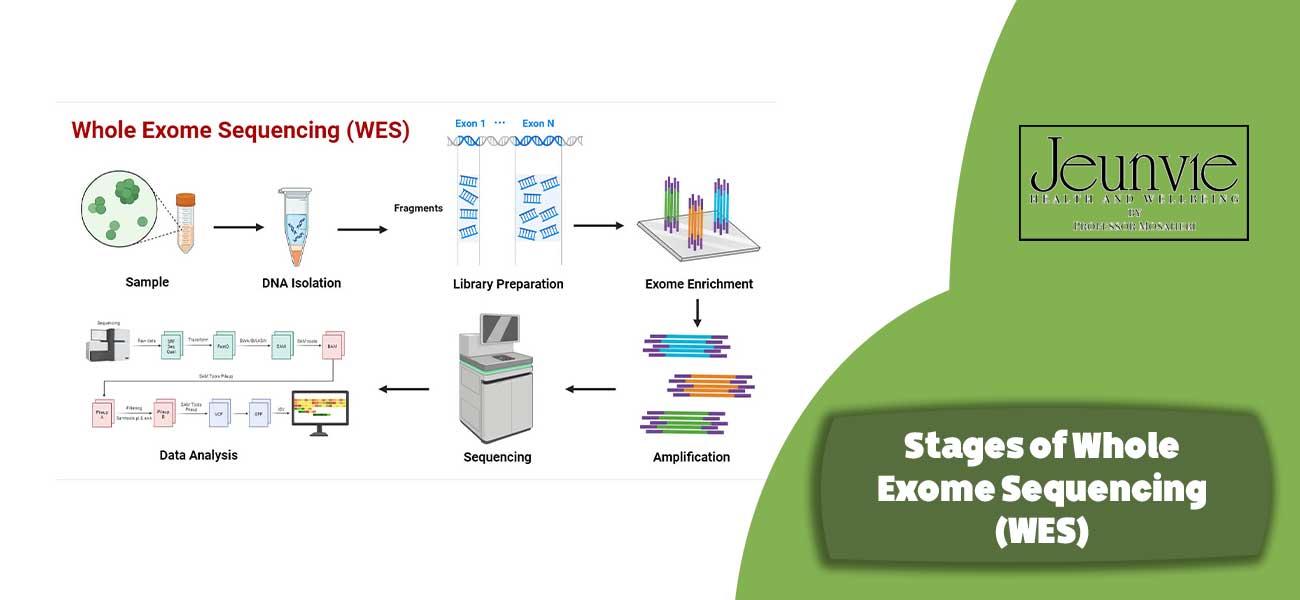
Stages of Whole Exome Sequencing (WES)
Whole Exome Sequencing (WES) is a multi-step process involving sample collection, preparation, sequencing, and data interpretation. The key stages of this process are outlined below:
DNA Sample Collection
The initial step in WES involves collecting a DNA sample from the patient. This sample is typically extracted from blood or tissue. Careful measures are taken to ensure sample quality and prevent contamination. The sample must be promptly transported to the laboratory to guarantee timely processing.
DNA Preparation and Library Construction
In this stage, the extracted DNA is prepared and converted into a library. This process includes the following steps:
- DNA Fragmentation: The DNA is broken down into smaller fragments (usually 200-300 base pairs) to facilitate analysis.
- Adapter Ligation: Specific sequences (adapters) are added to the ends of the DNA fragments to enable their identification and separation in subsequent sequencing steps.
- Amplification: If necessary, the DNA fragments are selectively amplified to achieve a sufficient concentration for sequencing.
Sequencing and Data Analysis
Once the DNA library is prepared, the sequencing stage commences. In this stage:
- Sequencing: Advanced methods, such as Next-Generation Sequencing (NGS), are employed to read the DNA sequence and identify exomes. These techniques allow for the simultaneous reading of millions of DNA fragments.
- Data Analysis: The obtained data is analysed using specialised software and algorithms to identify variations and mutations in the sequence. This stage involves assessing data quality and correcting any potential errors.
Result Interpretation and Variant Identification
After data analysis, the results must be interpreted. In this stage:
- Variant Identification: Genetic variations are identified by comparing the sequence to a reference sequence. This includes the identification of specific mutations, changes in exomes, and genetic abnormalities.
- Clinical Interpretation: Clinicians and researchers must evaluate the results in the context of the patient’s clinical condition. This interpretation aids in disease diagnosis, risk assessment, and treatment recommendations.
Overall, the WES process demands a high level of precision and expertise. To ensure accurate and meaningful results, it must be carried out meticulously. WES can significantly contribute to the diagnosis of inherited diseases, cancer diagnosis, and the design of personalised treatments.
Click to read this article : Genetic counseling
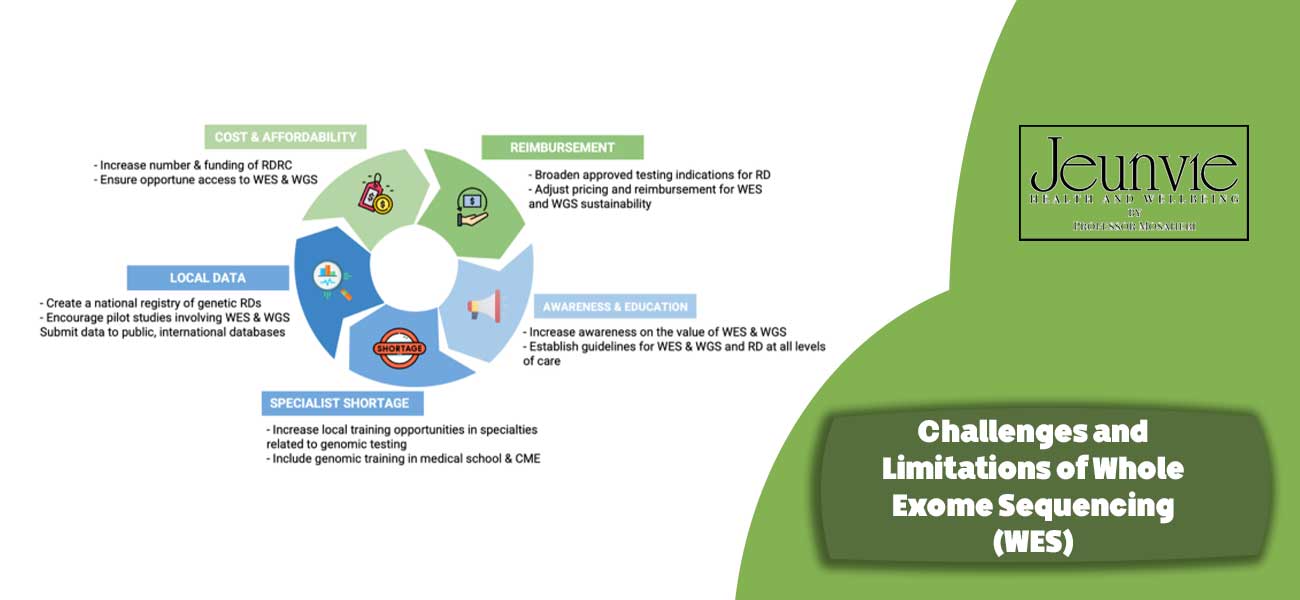
Challenges and Limitations of Whole Exome Sequencing (WES)
While Whole Exome Sequencing (WES) is a powerful technique with numerous benefits in diagnosing genetic diseases and personalising treatments, it also faces several challenges and limitations. These challenges are outlined below:
Sequencing Coverage Limitations
One of the primary challenges of WES is the limitation in sequencing coverage. This implies that some exons may not be fully covered, particularly in the following situations:
- Sample Quality: If the quality of the DNA sample is suboptimal (for instance, in bacterial samples or thin tissues), it may not be possible to accurately sequence all exonic regions.
- Complex Regions: Specific areas of the genome that contain long repeats or intricate sequences may not be well-characterised, which can lead to the loss of important information.
Data Interpretation Challenges
Interpreting the results of WES can be challenging, especially in the following scenarios:
- Variants of Unknown Significance: Many identified variants may be novel and lack sufficient information regarding their clinical implications. This can result in misinterpretation or a lack of interpretation altogether.
- Benign Variants: Some genetic variations may not be associated with any disease, and distinguishing these from pathogenic variants can be particularly difficult. This challenge may lead to uncertainty in treatment decisions.
High Costs
Conducting WES and analysing the resulting data can be costly. These expenses include:
- Laboratory Costs: The equipment and consumables required for WES and associated processes can incur significant costs.
- Data Analysis: Analysing the generated data necessitates specialised software and analytical expertise, which can add further costs.
In summary, although WES is a powerful tool in genetic science, its challenges and limitations necessitate caution and a comprehensive understanding of the processes and data interpretation. Continued research and methodological advancements can help alleviate these challenges and enhance the effectiveness of WES.

The Future of WES and Innovations
The future of Whole Exome Sequencing (WES) appears promising, with its applications expected to expand as technological advancements continue and our understanding of genetics deepens. Below are some key aspects of the future of WES and the associated innovations:
Emerging Technologies in DNA Sequencing
Developments in DNA sequencing technologies are ongoing. These include:
- Next-Generation Sequencing (NGS): This enhanced technology has significantly increased the speed and accuracy of sequencing, enabling researchers to generate more genetic data in a shorter time frame.
- DNA-free Sequencing: New technologies, such as tomography, aim to identify genetic changes without the need for DNA samples. This could facilitate broader applications of WES in clinical settings and research.
Improved Accuracy and Reduced Costs
With advancements in technology and novel data analysis methods, improvements in the accuracy of WES and significant reductions in costs are anticipated:
- Enhanced Accuracy: Advanced algorithms and analytical software can improve the precision of identifying genetic variations and reduce potential errors in result interpretation.
- Lower Costs: The development of new technologies and process optimisation is driving down the costs associated with WES, making this technique more accessible to researchers and clinicians alike.
Wider Adoption in Routine Medicine
WES is expected to see increased use as a diagnostic tool in everyday medical practice:
- Early Diagnosis: WES will enable clinicians to identify genetic diseases and assess individual risks at an earlier stage, potentially leading to improved treatment outcomes.
- Personalised Medicine: WES facilitates the creation of personalised treatments, allowing clinicians to provide therapies tailored to patients’ genetic profiles.
In summary, the future of WES, driven by technological advancements and decreasing costs, holds great promise for revolutionising the diagnosis and treatment of genetic diseases. As this technique becomes more widely adopted in routine medical practice, it is expected to contribute significantly to public health and the effectiveness of treatments.
Click to read this article : Hereditary cancer testing
Conclusion
Whole Exome Sequencing (WES), as an advanced tool in medical genetics, plays a crucial role in the diagnosis and treatment of inherited diseases. By focusing on the coding regions of the genome, WES facilitates the identification of rare and inherited mutations, which can lead to timely and effective treatments.
Significance of WES:
- Accurate Diagnosis: WES contributes to the identification of rare diseases and provides more precise information regarding mutations.
- Personalised Medicine: This method enables the tailoring of treatments based on each patient’s unique genetic profile.
- Innovative Research: WES assists researchers in understanding the impact of genes on disease development and in developing novel therapies.
In summary, WES stands as a vital asset in the realm of medical genetics, with its significance continuing to grow as technology advances and our understanding of genetics deepens.
Reviews
Contact
- Our Clinic : 4th Floor, 93-95 Wardour Street W1F 0UD
- phone: 07843 055357
- email:info@jeunviewellbeing.health
- Copyright 2025 Jeunvie Health and Wellbeing
